Lead-acid batteries play a prominent role in reliable and affordable energy storage. They have vast applications in cars and backup power systems. So, it is essential to know about it in depth. So, let’s discuss its composition, functions, and how the lead acid batteries work. Moreover, we will discuss some common issues with batteries and their solutions.
Part 1. What is a lead acid battery?

These batteries are composed of sulphuric acid electrolytes and lead. The lead is on electrodes, so one is positively charged (cathode), and the other is negatively charged (anode). These lead plates are submerged in the electrolytic solution, initiating the chemical reaction. So, this chemical reaction results in electrical energy (discharging). The reverse process of conversion of electrical power to chemical energy is the charging of the lead-acid battery. This is why it is known as a secondary battery.
Moreover, lead acid batteries work because of a low energy-to-volume ratio and lower energy-to-weight ratio. But it has a high power-to-weight ratio. Because it can supply a high surge voltage
Part 2. Sealed lead-acid battery vs lead-acid battery
A Sealed Lead Acid Battery (SLA) is designed explicitly with sealed construction. So they can resist electrolyte leakage. Here is a detailed table that will help you understand sealed lead-acid batteries. Moreover, it will tell you how sealed lead-acid batteries are a different and more suitable option.
| Feature | Lead Acid Battery | Sealed Lead Acid Battery (SLA) |
|---|---|---|
| Maintenance | It needs periodic maintenance such as checking electrolyte levels and topping up with distilled water. | Maintenance-free; sealed construction reduces the need for electrolyte checks or top-ups. |
| Electrolyte | Electrolyte is a liquid sulfuric acid solution. | The electrolyte is immobilized in a gel or absorbed in a glass mat, preventing leakage. |
| Design | Typically has vent caps to release gases produced during charging. | Sealed construction prevents leakage and emission of gases. |
| Mounting Options | Can be mounted in any orientation, but care must be taken to avoid electrolyte spillage. | Versatile mounting options due to sealed construction; can be mounted in various orientations without leakage concerns. |
| Lifespan | Generally shorter lifespan due to potential electrolyte evaporation and corrosion. | Longer lifespan due to sealed construction and reduced risk of electrolyte loss. |
| Usage | It is commonly used in applications where periodic maintenance is feasible. It includes automotive starting batteries. | It is best for maintenance-free operations such as UPS systems and emergency lighting. |
Part 3. What materials are used for lead-acid battery
So, before discussing composition and another working process, let’s discuss the materials used for lead-acid batteries.
- Lead (Pb): Lead is a primary component. It is used in both the battery’s positive and negative electrodes (plates). Moreover, it provides the conductivity and structure for the electrochemical reactions.
- Lead Dioxide (PbO2): it is only used in positive plates and works as an active material during discharging. Moreover, it speeds up the conversion of chemical energy into electrical energy.
- Sulfuric Acid (H2SO4): It is present in the electrolyte of lead-acid batteries with a water-to-acid ratio = 3:1. Moreover, it increases the flow of ions in the positive and negative electrodes.
- Plastic Casing: All battery components are covered inside a plastic casing. This casing provides insulation, protection, and structural stability to the battery.
Part 4. How do the lead acid batteries work?
So, people usually ask, how do the Lead Acid batteries work? Just like other batteries, lead batteries work due to the conversion of chemical energy into electrical energy. It is composed of lead plates. These plates are immersed in an electrolytic solution named sulphuric acid. So, chemical reactions start when you connect the lead-acid battery to a circuit. This results in the flow of electrons. These electrons travel from a negative plate to a positive plate. Sulphuric acid plays a bridging role in the movement of these ions and produces electrical energy. So, let’s discuss each phenomenon of the battery in sequence.
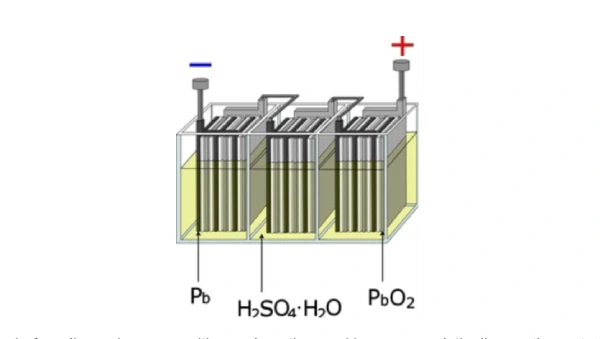
1. Chemical Reaction for Discharging
So, when the battery discharges, the following chemical reactions occur.
At the Positive Plate (Lead Dioxide)
PbO2 + H2SO4 + 2H^+ + 2e^- ————> PbSO4 + 2H2O
At the Negative Plate (Spongy Lead)
Pb + H2SO4 ——–> PbSO4 + 2H^+ + 2e^-
So, collectively, the net reaction during the discharging process is.
PbO2 + Pb + 2H2SO4 ——-> 2PbSO4 + 2H2O
So, the electrons released during this chemical reaction eventually generate electricity.
2. Chemical Reaction for Recharging
When the battery Recharges, the battery undergoes the following reactions,
At the Positive Plate (Lead Dioxide)
PbSO4 + 2H2O + 2e^- —— > PbO2 + 4H^+ + SO4^2-
At the Negative Plate (Spongy Lead)
PbSO4 + 2H^+ + 2e^- ———> Pb + H2SO4
Collectively it is,
2PbSO4 + 2H2O ——> PbO2 + Pb + 2H2SO4
So, the car alternator provides energy to carry out these reactions. They convert electrical energy back into Chemical energy stored in the battery.
Part 5. Types of lead-acid batteries
Lead-acid batteries have been in use since 1859. They are rechargeable and work as backup power for the vehicles. These batteries are relatively cheap, efficient, and have the simplest composition. So, there are different variations of lead acid battery work available. Let’s discuss all the types one by one.
1. Flooded Lead-Acid Batteries (FLA)
FLA is the most traditional and commonly used type of lead-acid battery. It consists mainly of lead plates. These plates are immersed in a liquid electrolyte named sulfuric acid. These batteries need regular maintenance. So, check and refill the electrolyte levels with distilled water. Furthermore, these batteries are widely used where the cost is the primary concern, i.e., automotive starting batteries.
2. Sealed Lead-Acid Batteries (SLA)
As their name suggests, sealed lead-acid batteries are sealed. So they prevent electrolyte leakage. Moreover, they use a gel or immobilized electrolyte. It keeps it in place. SLA batteries do not need maintenance. They do not require water to refill as well. These batteries have wide applications where maintenance is difficult, i.e., backup power supply for computer and alarm systems.
3. Gel Lead-Acid Batteries
These batteries use a thickening agent. This agent helps them immobilize the electrolyte and forms a gel-like substance. This gel substance prevents spillage and mounts the battery in different positions without leakage. Moreover, gel batteries have deep cycle capabilities. They are resistant to vibration and shock. So, they have wide applications in off-grid solar energy systems and electric wheelchairs.
4. Absorbent Glass Mat (AGM) Lead-Acid Batteries
AGM Lead acid batteries work with a fiberglass mat. It absorbs and immobilizes the electrolyte, allowing efficient oxygen recombination. Moreover, it reduces water loss and extends the battery life. Not only this, but AGM batteries also do not require maintenance, and they can be installed in any orientation without leakage. They are commonly used in high-performance applications like the RV, marine, and aviation industries.
Part 6. Classification of lead batteries based on usage
After discussing how the Lead Acid batteries work? Let’s go towards its classifications. Lead-acid batteries are reliable and versatile. They serve multiple purposes in different industries. If we talk about their usage, they are classified into two primary batteries.
1. Starter Batteries
Starter batteries are cranking or automotive batteries. They are designed to give energy to start the engine swiftly. These contain thin lead plates with high surface area. This is why they help optimize the current flow in short durations. These batteries prefer power density to energy capacity. Moreover, they enable rapid energy release to crank engines.
These batteries have wide applications in automotive vehicles, motorcycles, and other internal combustion engines.
2. Deep-cycle Batteries
These batteries are specifically designed to discharge a large portion of capacity. They tend to maintain the structural integrity and performance of various discharge cycles. Deep-cycle batteries have thicker lead plates. These plates are capable of withstanding frequent discharges without degradation. Moreover, they prefer energy capacity and longevity to power density. This feature helps them to sustain for a long time.
Part 7. Lead acid or li-ion battery: Which is better for your car?
Lithium-ion and lead acid batteries work for the same purpose in cars. But both have distinct features. They both have different characteristics. So, here is a short difference table for both batteries.
| Aspect | Lead Acid Battery | Li-ion Battery |
|---|---|---|
| Cost | Generally cheaper upfront | Initially more expensive, but may offer better value over time |
| Availability | Widely available, commonly used in cars | Becoming more common, but not as widespread as lead acid |
| Maintenance | Requires regular maintenance (topping up, cleaning) | Low maintenance |
| Weight | Heavier | Lighter |
| Performance | Provides consistent power output | Higher energy density, potentially better performance |
| Lifespan | Shorter lifespan compared to Li-ion | Longer lifespan |
We can say that both have their pros and cons. So, what you need is to go with what suits you best.
Part 8. What is the capacity of Lead-acid batteries?
The capacity of lead-acid batteries varies with their types and applications. So, here are some capacity ranges of different types with their applications.
| Aspect | Lead Acid Battery | Li-ion Battery |
|---|---|---|
| Cost | Generally cheaper upfront | Initially more expensive, but may offer better value over time |
| Availability | Widely available, commonly used in cars | Becoming more common, but not as widespread as lead acid |
| Maintenance | Requires regular maintenance (topping up, cleaning) | Low maintenance |
| Weight | Heavier | Lighter |
| Performance | Provides consistent power output | Higher energy density, potentially better performance |
| Lifespan | Shorter lifespan compared to Li-ion | Longer lifespan |
Part 9. Pros and Cons of Lead Batteries
Here is a comparison of the advantages and disadvantages of lead-acid batteries.
| Advantages | Disadvantages |
|---|---|
| Relatively low cost | Limited cycle life |
| Mature and well-established technology | Heavyweight |
| Wide availability and compatibility | Susceptible to sulfation |
| High starting power | Requires periodic maintenance |
| Tolerant to overcharging | Slow charging compared to some other battery types |
| Can provide high surge currents for starting engines | Limited deep discharge capability |
| Recyclable and environmentally friendly | Lead and sulfuric acid pose environmental concerns |
Part 10. Causes of Lead-Acid Battery Failure
However, lead acid batteries work for various industries. But every rose has its thorns. So, there are some failures associated with lead-acid batteries.
Partial Discharge
It happens when you do not completely discharge or recharge your battery. It leads to the sulfation of lead plates. This sulfation can create crystals over the lead plate and reduce the ability of the battery to store a charge. So, it can affect the overall performance of the battery.
Deep Discharge
It occurs when a battery discharges to a low voltage, i.e., lower than the recommended value. It can cause irreversible damage and result in the formation of hard sulfate crystals. These crystals are difficult to remove. Hence, they reduce the battery’s lifespan and lead to premature failure.
Overcharging
Overcharging also results in battery failure. Generally, it happens when you charge the battery for an extended period. It causes excessive gassing, plate corrosion, and thermal runaway. Moreover, it also damages the electrolyte and reduces the battery’s lifespan.
Part 11. How to Dispose of Spent Lead-Acid Batteries
It is important to dispose of the batteries after use. It leads to environmental safety. So, here are some regulations you can use to dispose of the batteries properly.
- First, check the local regulations and follow them.
- Different recycling platforms, battery retailers, and centers accept spent lead-acid batteries. So, you can bring them to these centers.
- Lead and sulphuric acid are hazardous materials. So, make sure to handle them with care.
- If you must shift these spent batteries to some location. You should transport them safely.
- Like other batteries, lead-acid batteries are recyclable. So, recycle them instead of piling them in one place or disposing them.
Part 12. Alternatives of Lead-acid Batteries
If we keep the limitations of lead acid batteries in mind, we also have different options in the market. These can lead us to better performance at optimizing results. So, here are some names of alternatives,
- Lithium-Ion Batteries
- Nickel-Metal Hydride (NiMH) Batteries
- Lithium Iron Phosphate (LiFePO4) Batteries
- Flow Batteries
- Lead-Carbon Batteries
Each of these has its pros and cons. You can check their properties and opt for the best solution.
Part 13. Conclusion
In conclusion, lead acid batteries work as one of the most reliable and cost-effective power solutions for vehicles. It is rechargeable and serves as backup power for various car electrical systems. It has wide applications in the market, such as RVs, marine vehicles, motorcycles, and other internal combustion systems. Although it has multiple advantages, but it offers some limitations as well. So, you can go for other batteries to fulfill your needs.
-
When is a Lead-acid Battery Charged?
It charges when an external current is applied to the battery. The source of this external current can be a charger or a car’s alternator. So, the reversible electrochemical reaction occurs inside the battery during the charging process. Here, the lead sulfates into lead dioxide at the positive lead plate. On the other side, lead sulfate converts into a metallic lead at the negative plate. This process stores the energy inside the battery to be used in the future. -
When Does the Self-Discharge of Lead-acid Battery Occur?
Suppose you do not connect the battery to any external source of current. In that case, it starts to lose its stored energy gradually. It is known as self-discharging. Several factors contribute to self-discharge. These may include internal resistance, temperature, and imbalance in the initial stage of the battery. Self-discharging is the natural function of lead-acid batteries. It helps to retain the charges for a long time. -
What type of lead-acid battery is it? Dry or Wet
It comes in both ways. Lead-acid batteries are of different kinds. A few of them are wet cells, such as flooded gel and absorbed glass mats (AGMs). While a few of the lead-acid batteries are dry cells. It includes sealed batteries. These batteries are commonly used in maintenance-free areas.
Related Tags:
More Articles

Overview of Deep Cycle Lithium Battery
In this article, we explore the life, voltage, capacity, and charging considerations of deep cycle lithium batteries.
How Long do Lithium Batteries Last?
How long do lithium batteries last? we will explore the factors that influence the lifespan of lithium batteries and provide insights into their longevity.
How to Choose the Best LiFePO4 Battery?
Choose LiFePO4 batteries for superior performance, safety, and versatility in EVs, UPS, and backup power. This guide helps you make informed decisions.
Get 12v Lithium Car Battery As a Power Source for the Ride
Make the right choice for your vehicle's battery needs by installing a 12 volt lithium car battery. You will enjoy maintenance-free longevity with this change.
Everything About A Small Lithium Ion Battery
Discover the features, uses & future potential of a small lithium ion battery. A compact and tiny powerhouse ideal for smartphones, wearables, drones & more.





